Which One of the Following Is a Decomposition Reaction
Fe CuSO 4. AAgCl 2 Ag Cl 2 b.

Combination And Decomposition Reaction Video Khan Academy
Which of the following statements concerning types of reactions is correct.

. CO 2 2 H 2 O c. CH 4 2O 2. CH4 g O2 g CO2 g H2O l 2.
Decomposition can be a single. - When it is heated strongly it starts to turn yellow and decompose carbon dioxide gas. Which of the following are decomposition reactions.
Experts are tested by Chegg as specialists in their subject area. Which of the following reactions is a decomposition reaction. D more than one correct response E no correct response.
We review their content and use your feedback to keep the quality high. Which one of the following is an example of decomposition reaction. 1 CH4 g O2 g CO2 g H2O l 2 CaO s CO2 g CaCO3 s 3 Mg s O2g MgO s 4 PbCO3 s PbO s CO2 g A 1 2 and 3 B 4 only C 1 2 3 and 4 D 2 and 3 E 2 3 and 4.
Which of the following is the best explanation for why this reaction is a decomposition reaction. B The reactants in a combination reaction must be elements. SO2 O2 - 2 SO3 B.
Option C is an example of a decomposition reaction. Which of the following is a decomposition reaction. Hence this is decomposition reaction.
Is decomposed into smaller fragments CaO and CO 2. A The reactant in a decomposition reaction must be a compound. Memorize flashcards and build a practice test to quiz yourself before your exam.
Who are the experts. N a O H H C l N a C l H 2 O is a displacement reaction. Decomposition reactions can be classified as either exothermic or endothermic depending on whether they release or absorb heat during the reaction.
Mg s O2 g MgO s 4. Chemical decomposition analysis or breakdown is the separation of a chemical compound into elements or simpler compounds. A decomposition reaction is one in which a reactant breaks down into simpler products.
See answers 3 Best Answer. Dec 29 2019. Cl2g NaBrs NaCIs Br2g d.
COg O2g CO2g c. Decomposition reaction - Separation of a substance into two or more substances that may differ from each other and from the original substance. A decomposition reaction occurs when one reactant breaks down into two or more products.
Which of the following is a decomposition reaction. O NH42CO3 -- NH3 H2O CO2 O Cd PbNO32 CdNO32 Pb O 2 HCl MgOH2 -- 2 H2O MgCl2 O Na2S CuNO32 -- 2 NaNO3 Cus O 2 Na Cl2 -- 2 Naci Question 6 Which of the following is a single replacement reaction. Which of the following reactions is a decomposition reaction.
Hence the correct option is E. Zinc carbonate is a white powdery solid. CaCO 3 heat.
2C 8 H 18 25O 2 16CO 2 18H 2 O. O B Ol c. Decomposition reactions are also classified by the number of steps required.
Start studying the Chemical Reactions Review flashcards containing study terms like Which of the following are decomposition reactions. Solve any question of Chemical Reactions and Equations with-. CACO3s CaOs CO2g O a O b O c none of the choices kindle DELE.
2KClO 3 2KCl3O 2. 100 6 ratings Refer. C The reactants in a single-replacement reaction could be two compounds.
Decomposition reaction - separation of a substance into two or more substances that may differ from each other and from the original substance. It is sometimes defined as the exact opposite of chemical synthesis. Examples of decomposition reactions include the breakdown of hydrogen peroxide to water and oxygen and the breakdown of water to hydrogen and oxygen.
5 Questions Show answers. H 2 SO 4 2NaOH Na 2 SO 4 2H 2 O. Which of the following is a decomposition reaction.
This can be represented by the general equation. The reaction for tarnish formation due to contact with hydrogen sulphire 2 A g g H 2 S g A g 2 S s H 2 g and the above tarnish can be removed by using A l metal as 2 A l 3 A g 2 S A l 2 S 3 6 A g The above two reactions appears to be a displacement reaction. CH1 O2g CO2g HO1 b.
2 H2O2 - 2 H2O O2 D. FeSO 4 Cu. AgNO3 NaCl - AgCl NaNO3.
View the full answer. 2HgO 2Hg O2. Zinc carbonate on heating decomposes to zinc oxide and carbon dioxide gas.
Which of the following is a decomposition reaction. 2 C2H2 5 O2 - 4 CO2 2 H2O C. The Correct Answer is Option C.
K2CO3 s K2O s CO2 g2SO3 g 2SO2 g O2 g. Is evolved which forms a white precipitate in limewater. CaO CO 2 d.
A decomposition reaction is a type of reaction where bonds are broken and new ones form. CaO s CO2 g CaCO3 s 3.

5 1 Synthesis And Decomposition Reactions Learning Goals Learn How To Identify A Chemical Change Learn What Is A Synthesis Reaction And How To Create Ppt Download
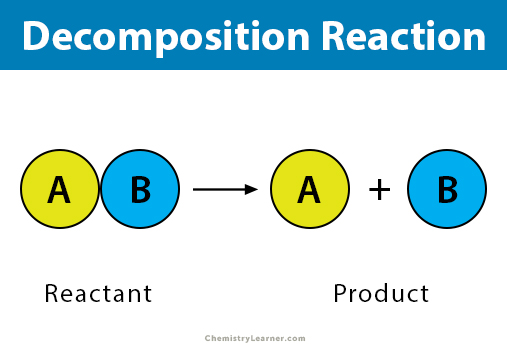
Decomposition Reaction Definition Examples Applications

Question 32 Assertion Reasoning Solutions Cbse Class 10 Sample

Which Of The Following Is Not A Thermal Decomposition Reaction Brainly In
Is Combustion A Thermal Decomposition Reaction Or A Combination Reaction Quora

Decomposition Reaction Read Chemistry Ck 12 Foundation

Which Of The Following Is Not A Thermal Decomposition Reaction According To The Picture Brainly In
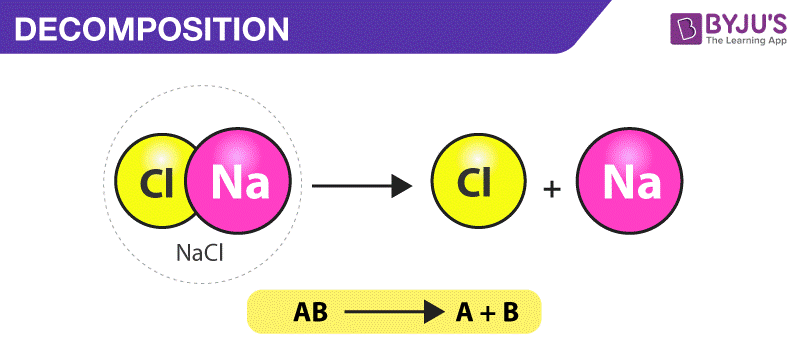
Decomposition Reaction Definition Types Examples Uses
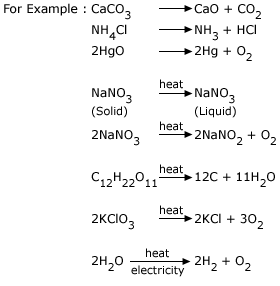
Breakage Of Bonds Formation Of Bonds

Chemical Reactions 4 Of 11 Decomposition Reactions An Explanation Youtube
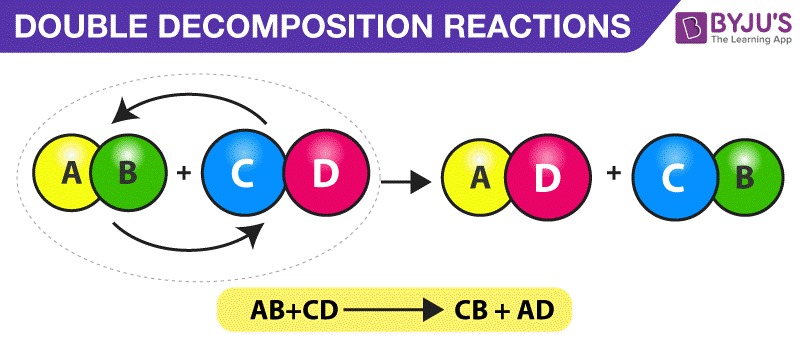
Decomposition Reaction Definition Types Examples Uses

5 1 Synthesis And Decomposition Reactions Learning Goals Learn How To Identify A Chemical Change Learn What Is A Synthesis Reaction And How To Create Ppt Download
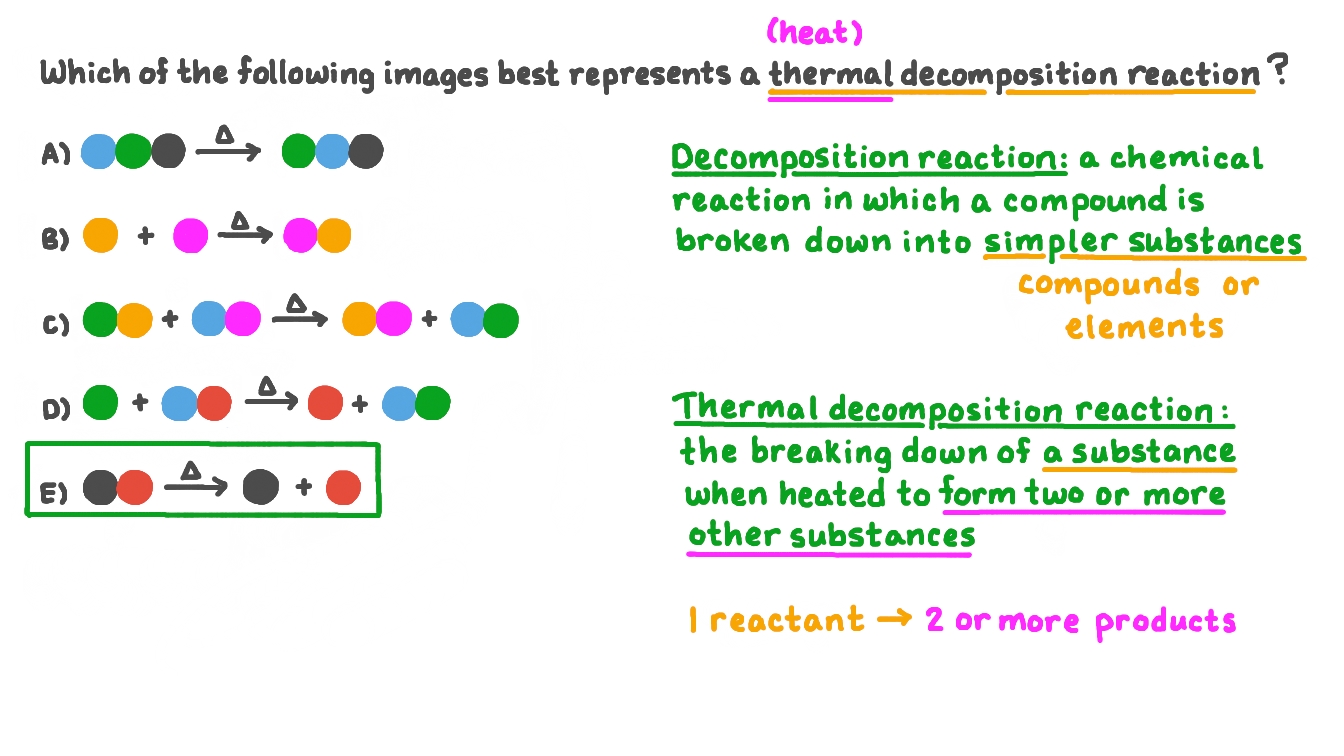
Question Video Identifying An Image That Represents A Thermal Decomposition Reaction Nagwa
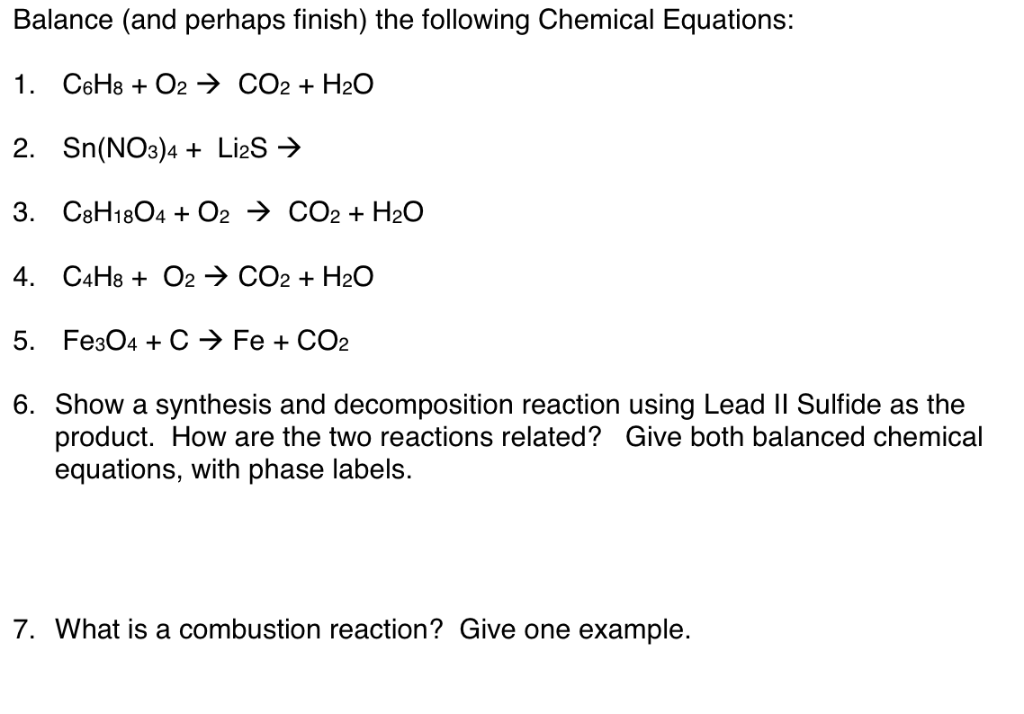
Solved Balance And Perhaps Finish The Following Chemical Chegg Com
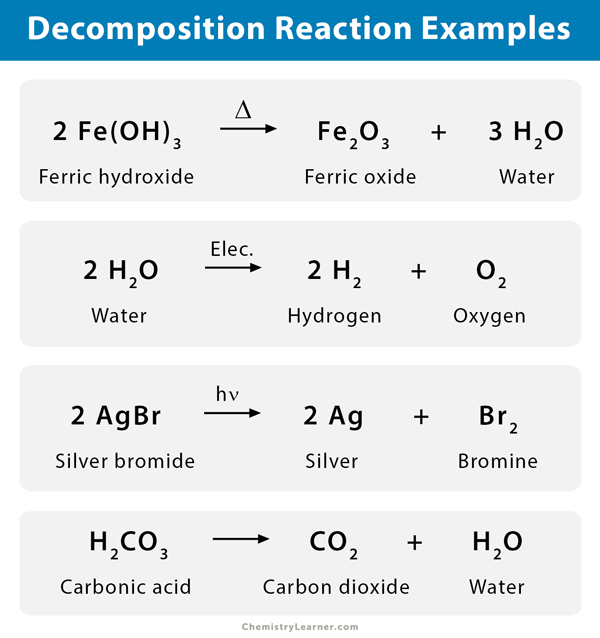
Decomposition Reaction Definition Examples Applications

What Is A Decomposition Reaction Definition And Examples

Chemical Reactions Combination Decomposition Combustion Single Double Displacement Chemistry Youtube

